When limestone (which is principally CaCO3)
is heated, carbon dioxide and quicklime
(Cao) are...

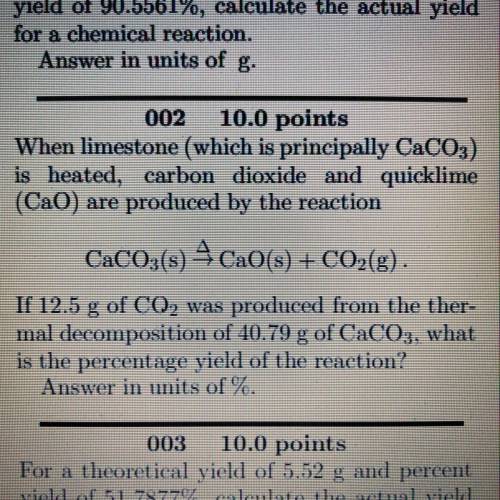
When limestone (which is principally CaCO3)
is heated, carbon dioxide and quicklime
(Cao) are produced by the reaction
CaCO3(s) 4CaO(s) + CO2(g).
If 12.5 g of CO2 was produced from the ther-
mal decomposition of 40.79 g of CaCO3, what
is the percentage yield of the reaction?
Answer in units of %.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
Questions

Chemistry, 01.02.2021 07:30

History, 01.02.2021 07:30




History, 01.02.2021 07:30


Mathematics, 01.02.2021 07:30

Chemistry, 01.02.2021 07:30

Mathematics, 01.02.2021 07:30

History, 01.02.2021 07:30

Computers and Technology, 01.02.2021 07:30

Biology, 01.02.2021 07:30

Mathematics, 01.02.2021 07:30

Mathematics, 01.02.2021 07:30


Mathematics, 01.02.2021 07:40


Mathematics, 01.02.2021 07:40

Chemistry, 01.02.2021 07:40



