
Chemistry, 04.03.2021 22:40 abrown6758
Suppose the students had conducted an additional trial in Experiment 2 using a buffer solution with a pH of 6.3. What would most likely be the results of the iodine and Benedict's tests?
A. Iodine TEST: Black Benedict's TEST: NCC
B. Iodine TEST: Black Benedict's TEST: Orange
C. Iodine TEST: NCC Benedict's TEST: Orange
D. Iodine TEST: NCC Benedict's TEST: NCC
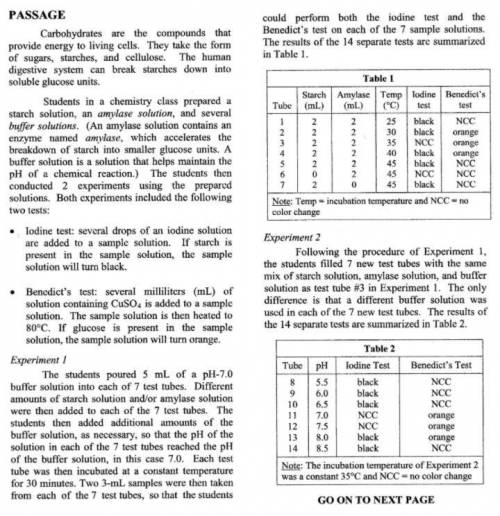

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
Suppose the students had conducted an additional trial in Experiment 2 using a buffer solution with...
Questions

Mathematics, 16.10.2021 20:50

English, 16.10.2021 20:50

Biology, 16.10.2021 20:50


Mathematics, 16.10.2021 20:50

History, 16.10.2021 20:50

Mathematics, 16.10.2021 20:50



Biology, 16.10.2021 20:50

Mathematics, 16.10.2021 20:50


History, 16.10.2021 20:50



English, 16.10.2021 20:50

Advanced Placement (AP), 16.10.2021 20:50

Mathematics, 16.10.2021 20:50


Mathematics, 16.10.2021 20:50



