
Chemistry, 04.03.2021 23:20 chicalapingpon1938
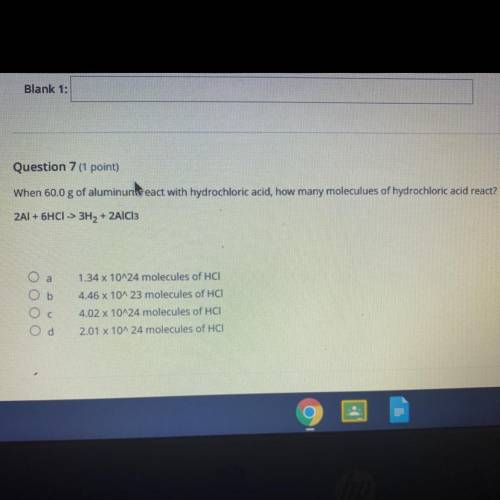
Question 7 (1 point)
When 60.0 g of aluminum react with hydrochloric acid, how many moleculues of hydrochloric acid react?
2AI + 6HCI -> 3H2 + 2AlCl3
O a
1.34 x 10^24 molecules of HCI-
Ob
Ос
4.46 x 10A 23 molecules of HCI
4.02 x 10^24 molecules of HCI:
2.01 x 10^24 molecules of HCI

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Question 7 (1 point)
When 60.0 g of aluminum react with hydrochloric acid, how many moleculues of h...
Questions

Mathematics, 13.12.2021 20:30


Mathematics, 13.12.2021 20:30




Health, 13.12.2021 20:30

Mathematics, 13.12.2021 20:30

Mathematics, 13.12.2021 20:30


Mathematics, 13.12.2021 20:30

History, 13.12.2021 20:30

Mathematics, 13.12.2021 20:30


English, 13.12.2021 20:30

English, 13.12.2021 20:30

Social Studies, 13.12.2021 20:30

Arts, 13.12.2021 20:30


Mathematics, 13.12.2021 20:30



