Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
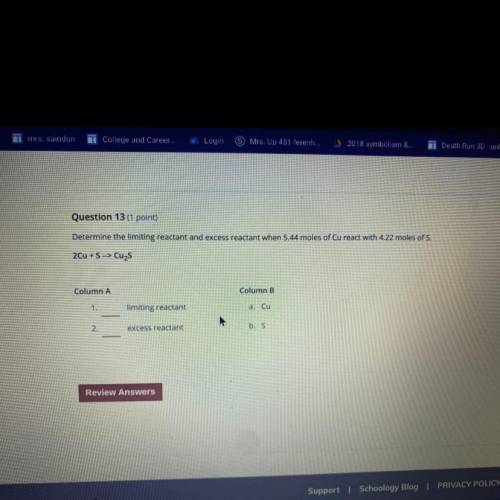
Question 13 (1 point)
Determine the limiting reactant and excess reactant when 5.44 moles of Cu rea...
Questions

Arts, 12.04.2021 06:10





Mathematics, 12.04.2021 06:10




Mathematics, 12.04.2021 06:10

Mathematics, 12.04.2021 06:10

Mathematics, 12.04.2021 06:10

Social Studies, 12.04.2021 06:10


Mathematics, 12.04.2021 06:10

History, 12.04.2021 06:10

Mathematics, 12.04.2021 06:10


Mathematics, 12.04.2021 06:10

Chemistry, 12.04.2021 06:10