Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
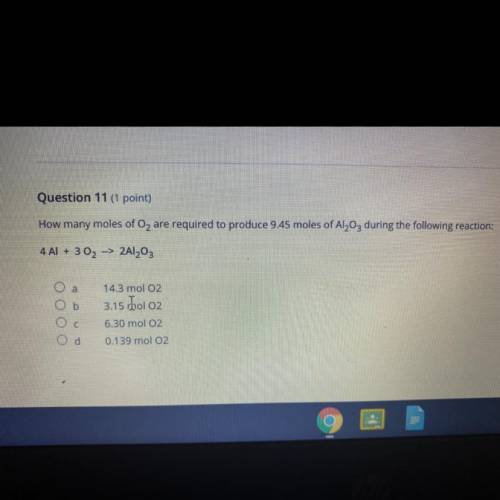
Question 11 (1 point)
How many moles of O2 are required to produce 9.45 moles of Al2O3 during the f...
Questions


Business, 08.11.2020 07:50


History, 08.11.2020 07:50

Mathematics, 08.11.2020 07:50

Mathematics, 08.11.2020 07:50

Mathematics, 08.11.2020 07:50


English, 08.11.2020 07:50



Mathematics, 08.11.2020 07:50

History, 08.11.2020 07:50

Social Studies, 08.11.2020 07:50

Mathematics, 08.11.2020 07:50


Mathematics, 08.11.2020 07:50

Physics, 08.11.2020 07:50

Mathematics, 08.11.2020 07:50

Arts, 08.11.2020 07:50