
Chemistry, 04.03.2021 23:40 kingjames82
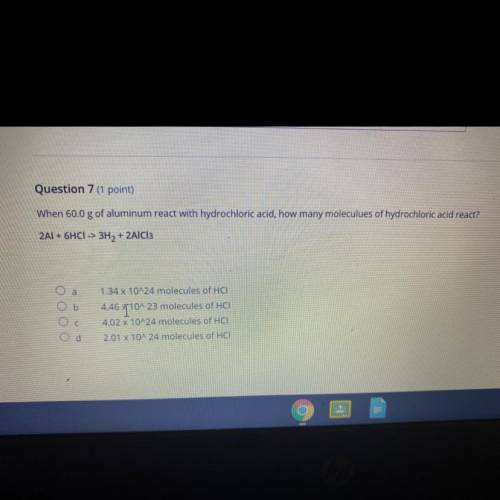
Question 7 (1 point)
When 60.0 g of aluminum react with hydrochloric acid, how many moleculues of hydrochloric acid react?
2Al + 6HCI -> 3H2 + 2AlCl3
a
b
ОООО
1.34 x 10^24 molecules of HCI
4.46 T10^23 molecules of HCI
4.02 x 10^24 molecules of HCI
2.01 X 10^24 molecules of HCI

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

You know the right answer?
Question 7 (1 point)
When 60.0 g of aluminum react with hydrochloric acid, how many moleculues of h...
Questions

English, 31.03.2021 04:20



Computers and Technology, 31.03.2021 04:20


Mathematics, 31.03.2021 04:20

History, 31.03.2021 04:20


Chemistry, 31.03.2021 04:20

Mathematics, 31.03.2021 04:20

English, 31.03.2021 04:20

Mathematics, 31.03.2021 04:20

Mathematics, 31.03.2021 04:20

Mathematics, 31.03.2021 04:20

Mathematics, 31.03.2021 04:20

Mathematics, 31.03.2021 04:20

English, 31.03.2021 04:20



History, 31.03.2021 04:20



