Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes or no?
Answers: 1
You know the right answer?
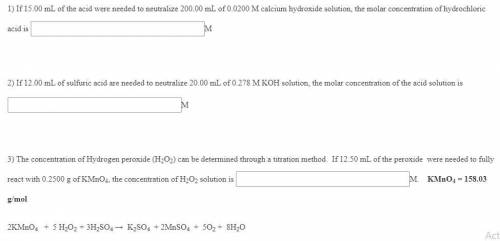
If 15.00 mL of the acid were needed to neutralize 200.00 mL of 0.0200 M calcium hydroxide solution,...
Questions

Biology, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Biology, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Social Studies, 14.09.2020 14:01

Biology, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

History, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Social Studies, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Social Studies, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

Mathematics, 14.09.2020 14:01

History, 14.09.2020 14:01