
Chemistry, 05.03.2021 02:40 chazpooh208
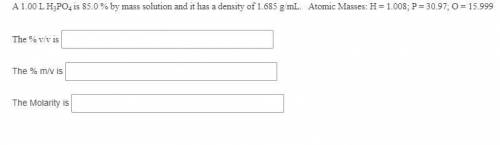
A 1.00 L H3PO4 is 85.0 % by mass solution and it has a density of 1.685 g/mL. Atomic Masses: H = 1.008; P = 30.97; O = 15.999

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
A 1.00 L H3PO4 is 85.0 % by mass solution and it has a density of 1.685 g/mL. Atomic Masses: H = 1.0...
Questions

Chemistry, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30

Physics, 30.06.2019 22:30



Biology, 30.06.2019 22:30



Mathematics, 30.06.2019 22:30

History, 30.06.2019 22:30

History, 30.06.2019 22:30


Mathematics, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30


Mathematics, 30.06.2019 22:30

Physics, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30

Mathematics, 30.06.2019 22:30




