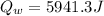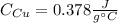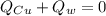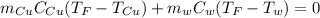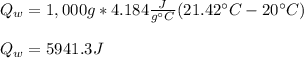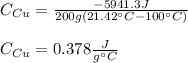Chemistry, 05.03.2021 04:30 ibrahimuskalel
Mix 200 g of copper at 100 °C with 1,000 g of water at 20 °C. Final temp. = 21.42°C a) How much heat energy (q) did the water gain? b) Now solve for the specific heat (c) of copper:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3


You know the right answer?
Mix 200 g of copper at 100 °C with 1,000 g of water at 20 °C. Final temp. = 21.42°C a) How much heat...
Questions

Mathematics, 16.12.2020 23:10


English, 16.12.2020 23:10

Mathematics, 16.12.2020 23:10


Mathematics, 16.12.2020 23:10

Biology, 16.12.2020 23:10


History, 16.12.2020 23:10

Mathematics, 16.12.2020 23:10

Mathematics, 16.12.2020 23:10

English, 16.12.2020 23:10



Mathematics, 16.12.2020 23:10


Business, 16.12.2020 23:10

Mathematics, 16.12.2020 23:10

Mathematics, 16.12.2020 23:10

History, 16.12.2020 23:10