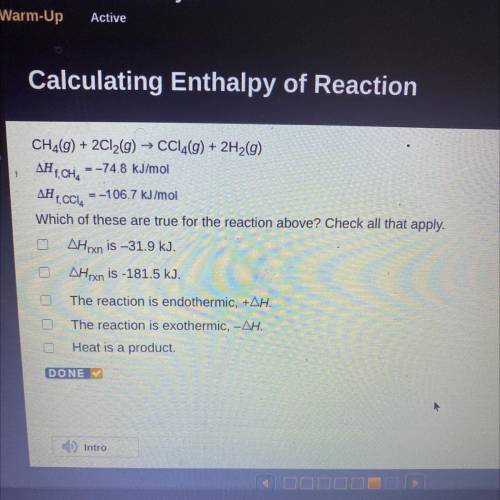
CH2(g) + 2Cl2(g) → CCl4(g) + 2H2(g)
AH T, CH, = -74.8 kJ/mol
AH 1. CCI, = -106.7 kJ/mol
...

Chemistry, 05.03.2021 07:00 HaydenSturgis1
CH2(g) + 2Cl2(g) → CCl4(g) + 2H2(g)
AH T, CH, = -74.8 kJ/mol
AH 1. CCI, = -106.7 kJ/mol
Which of these are true for the reaction above? Check all that apply.
AHrxn is –31.9 kJ.
AHrxn is -181.5 kJ.
The reaction is endothermic, +AH.
The reaction is exothermic, -AH.
Heat is a product.
PLEASE HELP IM BEGGING AND I WILL GIVE BRAINLIEST

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

You know the right answer?
Questions

Biology, 12.08.2020 08:01



Mathematics, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01



Mathematics, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01


Mathematics, 12.08.2020 08:01


English, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01

Social Studies, 12.08.2020 08:01

History, 12.08.2020 08:01



Mathematics, 12.08.2020 08:01



