
Chemistry, 05.03.2021 06:50 rleiphart1
When 1.50 mol CO2 and 1.50 mol H2 are placed in a 3.00-Lcontainer at 395 °C, the following reaction
CO2(g) + H2(g) = CO(g) + H2O(g). If Kc = 0.802,
what are the concentrations of each substance in the equilibrium mixture?
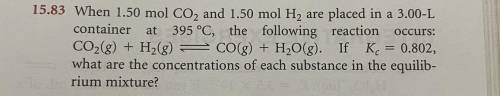

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

You know the right answer?
When 1.50 mol CO2 and 1.50 mol H2 are placed in a 3.00-Lcontainer at 395 °C, the following reaction...
Questions



Computers and Technology, 21.08.2019 20:20


Computers and Technology, 21.08.2019 20:20


Social Studies, 21.08.2019 20:20




Mathematics, 21.08.2019 20:30

Mathematics, 21.08.2019 20:30










