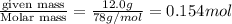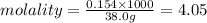Chemistry, 05.03.2021 16:00 carson5238
The concentration of a benzene solution prepared by mixing 12.0 g C6H6 with 38.0 g CCl4 is molal

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
The concentration of a benzene solution prepared by mixing 12.0 g
C6H6 with 38.0 g CCl4 is molal...
Questions

Mathematics, 10.12.2020 22:10

Mathematics, 10.12.2020 22:10

Mathematics, 10.12.2020 22:10


Mathematics, 10.12.2020 22:10




Chemistry, 10.12.2020 22:10



History, 10.12.2020 22:10





English, 10.12.2020 22:10

Mathematics, 10.12.2020 22:10


Mathematics, 10.12.2020 22:10

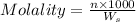
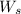 = weight of solvent in g
= weight of solvent in g
 =
=