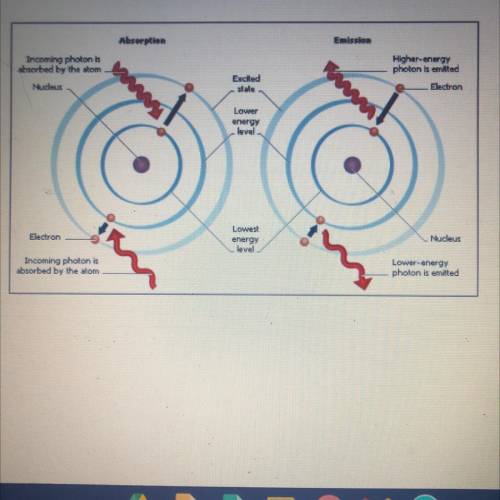
Q.1 When a metal is heated, what
happens to the electrons in
the atoms?
Q.2 What is th...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
Questions

Biology, 15.07.2019 03:20


History, 15.07.2019 03:20


Social Studies, 15.07.2019 03:20



Biology, 15.07.2019 03:20

Biology, 15.07.2019 03:20


Social Studies, 15.07.2019 03:20


Mathematics, 15.07.2019 03:20

Mathematics, 15.07.2019 03:20

History, 15.07.2019 03:20