N2 + 3H2 2NH3

Chemistry, 05.03.2021 19:40 kayliebug2003
(image is the layout of the table)
Let's look at the chemical equation again:
N2 + 3H2 2NH3
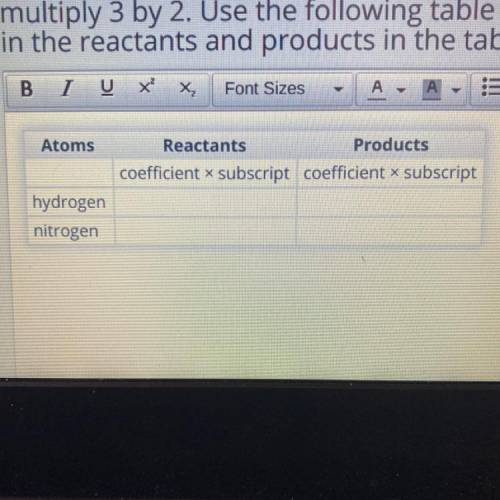
This time, you'll use a different method to count the atoms. Multiply the coefficient by the
subscript for each atom. For example, to find the number of hydrogen atoms in 3H2,
multiply 3 by 2. Use the following table to organize your work. Fill in the number of atoms
in the reactants and products in the table.
Atoms
Reactants
Products
coefficient x subscript coefficient x subscript
hydrogen
nitrogen

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
(image is the layout of the table)
Let's look at the chemical equation again:
N2 + 3H2 2NH3
N2 + 3H2 2NH3
Questions




Mathematics, 09.04.2021 23:00

Spanish, 09.04.2021 23:00





World Languages, 09.04.2021 23:00


Mathematics, 09.04.2021 23:00


Physics, 09.04.2021 23:00


Mathematics, 09.04.2021 23:00







