Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
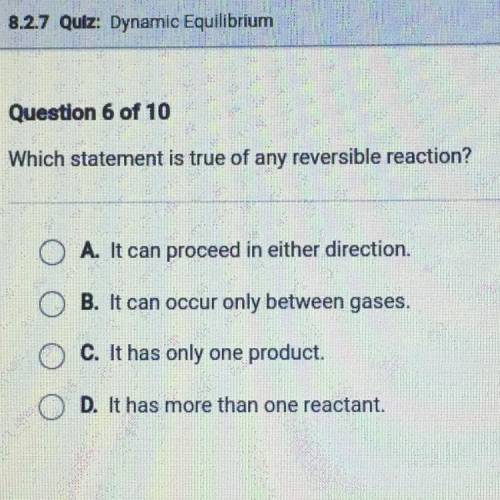
Which statement is true of any reversible reaction?
A. It can proceed in either direction
Questions

Biology, 29.12.2019 10:31






Mathematics, 29.12.2019 10:31

Mathematics, 29.12.2019 10:31

Geography, 29.12.2019 10:31


Social Studies, 29.12.2019 10:31

Mathematics, 29.12.2019 10:31


Mathematics, 29.12.2019 10:31

Mathematics, 29.12.2019 10:31


Mathematics, 29.12.2019 10:31

Social Studies, 29.12.2019 10:31

Computers and Technology, 29.12.2019 10:31

English, 29.12.2019 10:31