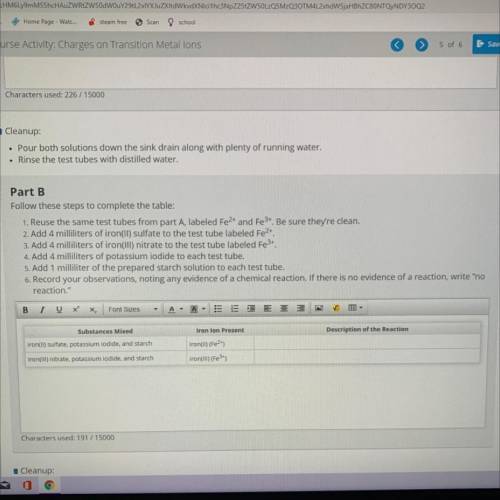
Part B
Follow these steps to complete the table:
1. Reuse the same test tubes from part A, la...

Chemistry, 05.03.2021 22:30 xxQueenPxx7432
Part B
Follow these steps to complete the table:
1. Reuse the same test tubes from part A, labeled Fe2+ and Fe3+. Be sure they're clean.
2. Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2+.
3. Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
4. Add 4 milliliters of potassium iodide to each test tube.
5. Add 1 milliliter of the prepared starch solution to each test tube.
6. Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a reaction, w
reaction."
B 1
U
x
x х.
Font Sizes
AA. EE
Substances Mixed
Iron lon Present
Description of the Reaction
iron(II) sulfate, potassium iodide, and starch
iron(ul) (Fe2+)
iron(III) (Fe 3+)
iron(III) nitrate, potassium iodide, and starch

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2


Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
Questions

Computers and Technology, 04.11.2020 19:20


Mathematics, 04.11.2020 19:20


Mathematics, 04.11.2020 19:20


Health, 04.11.2020 19:20




Mathematics, 04.11.2020 19:20


Biology, 04.11.2020 19:20





Computers and Technology, 04.11.2020 19:20


Mathematics, 04.11.2020 19:20



