(05.07 HC)
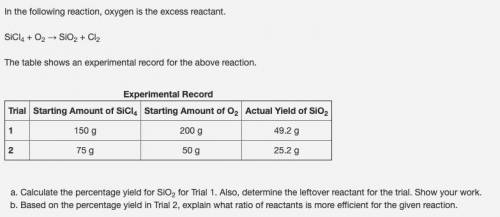
In the following reaction, oxygen is the excess reactant.
SiCl4 + O2 → SiO2...

(05.07 HC)
In the following reaction, oxygen is the excess reactant.
SiCl4 + O2 → SiO2 + Cl2
The table shows an experimental record for the above reaction.
Experimental Record
Trial Starting Amount of SiCl4 Starting Amount of O2 Actual Yield of SiO2
1 150 g 200 g 49.2 g
2 75 g 50 g 25.2 g
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work.
Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Questions

Mathematics, 25.10.2020 21:30

Mathematics, 25.10.2020 21:30


Mathematics, 25.10.2020 21:30



Mathematics, 25.10.2020 21:30


Computers and Technology, 25.10.2020 21:30

Mathematics, 25.10.2020 21:30



History, 25.10.2020 21:30

Mathematics, 25.10.2020 21:30


English, 25.10.2020 21:30

Mathematics, 25.10.2020 21:30

Biology, 25.10.2020 21:30

Social Studies, 25.10.2020 21:30

Mathematics, 25.10.2020 21:30



