
Chemistry, 06.03.2021 01:00 alecnewman2002
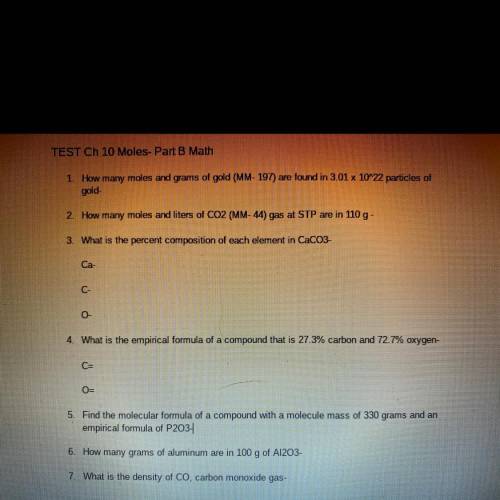
TEST Ch 10 Moles- Part B Math
1. How many moles and grams of gold (MM- 197) are found in 3.01 x 10^22 particles of
gold-
2. How many moles and liters of CO2 (MM-44) gas at STP are in 110 g -
3. What is the percent composition of each element in CaCO3-
Ca-
C-
0
4. What is the empirical formula of a compound that is 27.3% carbon and 72.7% oxygen-
C=
O-
5. Find the molecular formula of a compound with a molecule mass of 330 grams and an
empirical formula of P203-
6. How many grams of aluminum are in 100 g of A1203-
7. What is the density of Co, carbon monoxide gas-

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1


Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
TEST Ch 10 Moles- Part B Math
1. How many moles and grams of gold (MM- 197) are found in 3.01 x 10^...
Questions

Mathematics, 20.11.2020 01:40

Computers and Technology, 20.11.2020 01:40


Medicine, 20.11.2020 01:40

Mathematics, 20.11.2020 01:40




Mathematics, 20.11.2020 01:40

Mathematics, 20.11.2020 01:40


History, 20.11.2020 01:40


Mathematics, 20.11.2020 01:40


Physics, 20.11.2020 01:40

Mathematics, 20.11.2020 01:40



Mathematics, 20.11.2020 01:40



