
Chemistry, 06.03.2021 03:20 mariahcid904
Determine the molecular formula for the compounds below.
C2H4O ; molar mass=176 g/mol
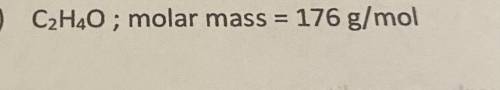

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Determine the molecular formula for the compounds below.
C2H4O ; molar mass=176 g/mol
...
C2H4O ; molar mass=176 g/mol
...
Questions


Biology, 01.08.2019 08:00



History, 01.08.2019 08:00


French, 01.08.2019 08:00



Biology, 01.08.2019 08:00

English, 01.08.2019 08:00



Health, 01.08.2019 08:00

Mathematics, 01.08.2019 08:00







