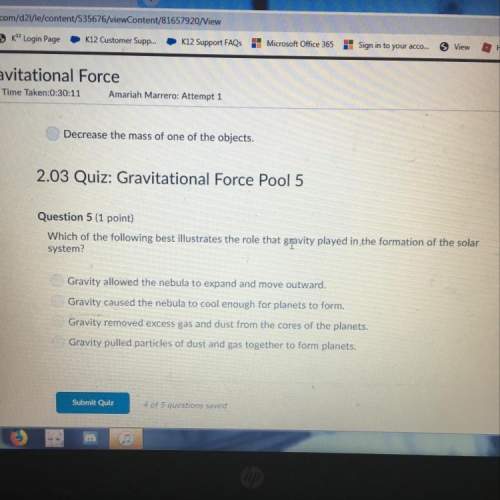
Chemistry, 06.03.2021 06:40 angeljohnson2081
A 10.0 ml aliquot of an acidic substance was titrated with a 0.05 M NaOH, requiring 30.5 ml to reach the
potentiometric end point. If the acidic substance has a density of
1.05 g/ml, calculate it's acidity as percent acetic acid Molecular weight of acetic acid is 60.0g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
A 10.0 ml aliquot of an acidic substance was titrated with a 0.05 M NaOH, requiring 30.5 ml to reach...
Questions

Chemistry, 04.11.2021 03:00

Social Studies, 04.11.2021 03:00

Mathematics, 04.11.2021 03:00

World Languages, 04.11.2021 03:00





Mathematics, 04.11.2021 03:10

History, 04.11.2021 03:10

Chemistry, 04.11.2021 03:10

Mathematics, 04.11.2021 03:10