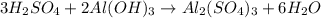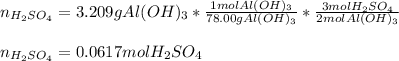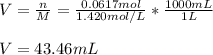Chemistry, 06.03.2021 08:00 jasminellenaee
What volume in milliliters of 1.420 M sulfuric acid is needed to neutralize 3.209 g of aluminum hydroxide 3 H 2 SO 4 (aq)+2 Al(OH) 3 (aq) Al 2 (SO 4 ) 3 (aq)+6 H 2 O

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
What volume in milliliters of 1.420 M sulfuric acid is needed to neutralize 3.209 g of aluminum hydr...
Questions


Biology, 20.11.2019 20:31

Geography, 20.11.2019 20:31


Mathematics, 20.11.2019 20:31

Biology, 20.11.2019 20:31

Mathematics, 20.11.2019 20:31


Mathematics, 20.11.2019 20:31


English, 20.11.2019 20:31




Social Studies, 20.11.2019 20:31

Mathematics, 20.11.2019 20:31




Social Studies, 20.11.2019 20:31