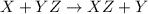Will the following reaction occur?
Mg + HCI →
o Yes, magnesium will replace hydrogen because...

Chemistry, 06.03.2021 08:10 rosie20052019
Will the following reaction occur?
Mg + HCI →
o Yes, magnesium will replace hydrogen because a bond between H-Cl is unstable.
Yes, magnesium will replace hydrogen because hydrogen is higher on the activity series
list.
o
o this reaction will not occur
Yes, magnesium will replace hydrogen because magnesium is higher on the activity
series list.
2 3 4 5 6
7
8
9 10 11

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
Questions



Computers and Technology, 22.03.2021 21:30



Mathematics, 22.03.2021 21:30



Chemistry, 22.03.2021 21:30

History, 22.03.2021 21:30

Mathematics, 22.03.2021 21:30

Mathematics, 22.03.2021 21:30

Chemistry, 22.03.2021 21:30




Mathematics, 22.03.2021 21:30

Mathematics, 22.03.2021 21:30

Computers and Technology, 22.03.2021 21:30