
Chemistry, 08.03.2021 01:30 4tazaouiamine1r
< Question 16 of 21 >
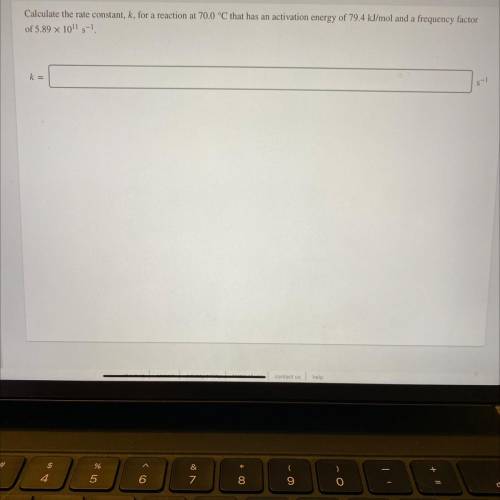
Calculate the rate constant, k, for a reaction at 70.0 °C that has an activation energy of 79.4 kJ/mol and a frequency factor
of 5.89 x 1011 5-1
s-1
k=

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
< Question 16 of 21 >
Calculate the rate constant, k, for a reaction at 70.0 °C that has an a...
Questions


English, 07.05.2021 05:20


Mathematics, 07.05.2021 05:20

Mathematics, 07.05.2021 05:20


Computers and Technology, 07.05.2021 05:20

Mathematics, 07.05.2021 05:20


Social Studies, 07.05.2021 05:20

Social Studies, 07.05.2021 05:20

Mathematics, 07.05.2021 05:20



Arts, 07.05.2021 05:20

Mathematics, 07.05.2021 05:20

Mathematics, 07.05.2021 05:20



English, 07.05.2021 05:20



