
Chemistry, 08.03.2021 14:00 24hudsonmoss
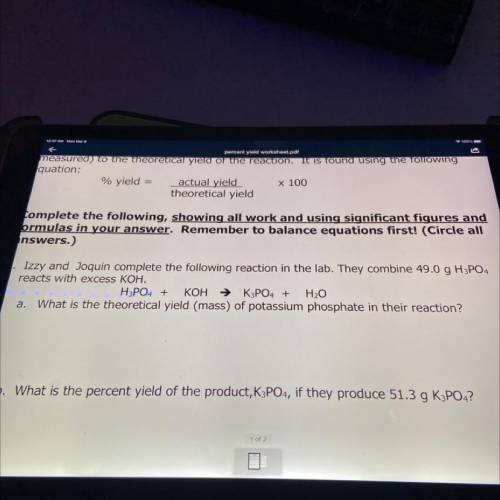
Izzy and Joquin complete the following reaction in the lab. They combine 49.0 g H3PO4
reacts with excess KOH.
H3PO4 + KOH → K3PO4 + H2O
a. What is the theoretical yield (mass) of potassium phosphate in their reaction?
b. What is the percent yield of the product, K3PO4, if they produce 51.3 g K3PO4?
1 of 2

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1



Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1
You know the right answer?
Izzy and Joquin complete the following reaction in the lab. They combine 49.0 g H3PO4
reacts with e...
Questions


Chemistry, 12.10.2019 02:00

Mathematics, 12.10.2019 02:00



Mathematics, 12.10.2019 02:00

English, 12.10.2019 02:00

Mathematics, 12.10.2019 02:00

Spanish, 12.10.2019 02:00

Biology, 12.10.2019 02:00

Mathematics, 12.10.2019 02:00

Mathematics, 12.10.2019 02:00





Mathematics, 12.10.2019 02:00

Mathematics, 12.10.2019 02:00

English, 12.10.2019 02:00

Computers and Technology, 12.10.2019 02:00



