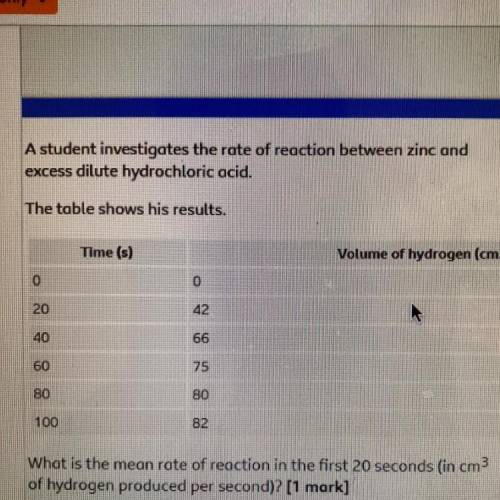
A student investigates the rate of reaction between zinc and
excess dilute hydrochloric acid.
...

Chemistry, 08.03.2021 14:00 roseemariehunter12
A student investigates the rate of reaction between zinc and
excess dilute hydrochloric acid.
The table shows his results.
Time (s)
Volume of hydrogen (cm3)
0
0
20
42
40
66
60
80
80
100
82

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
The skeletal system performs a variety of functions that are crucial to maintaining life processes. what function is performed in the bone marrow, but not in the ossified bones of the skeleton? a oxygen transportation c mineral storage b. muscle attachment d red blood cell production
Answers: 3


Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
Questions






History, 14.11.2019 06:31











Health, 14.11.2019 06:31





