
Chemistry, 08.03.2021 15:00 sihamabdalla591
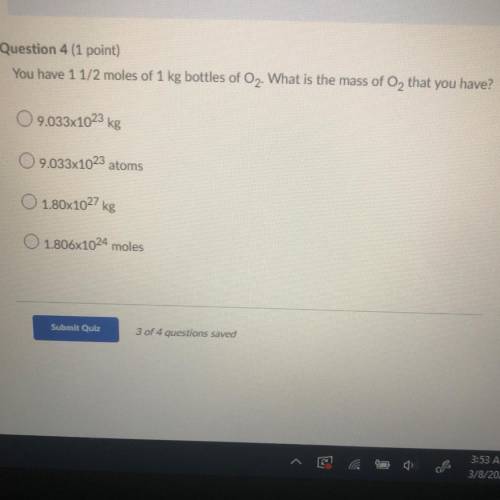
You have 1 1/2 moles of 1 kg bottles of O2. What is the mass of O2 that you have?
A. 9.033x10^23 kg
B. 9.033x10^23 atoms
C. 1.80x10^27 kg
D. 1.806x10^24 moles

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
You have 1 1/2 moles of 1 kg bottles of O2. What is the mass of O2 that you have?
A. 9.033x10^23 kg...
Questions

Mathematics, 03.02.2020 14:54

History, 03.02.2020 14:54

History, 03.02.2020 14:54

Mathematics, 03.02.2020 14:54


History, 03.02.2020 14:54



Mathematics, 03.02.2020 14:54


Mathematics, 03.02.2020 14:54



History, 03.02.2020 14:54

History, 03.02.2020 14:54



Mathematics, 03.02.2020 14:54

History, 03.02.2020 14:54

Mathematics, 03.02.2020 14:54



