Chemistry, 08.03.2021 20:10 alvaradovanessa14
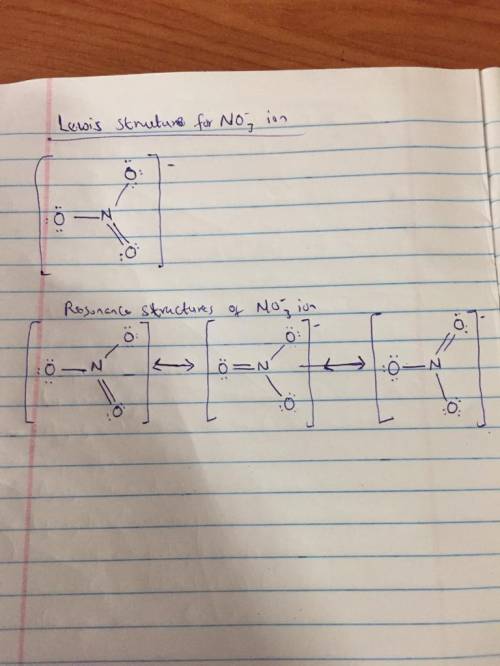
Discuss the nature of the bonding in the nitrate ion ( ) NO32 .Draw the possible Lewis resonance diagrams for this ion. Use the VSEPR theory to determine the steric number, thehybridization of the central N atom, and the geometry ofthe ion. Show how the use of resonance structures can beavoided by introducing a de-localized p MO. What bondorder is predicted by the MO model for the NUO bondsin the nitrate ion

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Discuss the nature of the bonding in the nitrate ion ( ) NO32 .Draw the possible Lewis resonance dia...
Questions


English, 05.05.2020 17:00



Chemistry, 05.05.2020 17:00





English, 05.05.2020 17:00

Physics, 05.05.2020 17:00


History, 05.05.2020 17:00

History, 05.05.2020 17:01



Computers and Technology, 05.05.2020 17:01

Mathematics, 05.05.2020 17:01

Mathematics, 05.05.2020 17:01

Biology, 05.05.2020 17:01