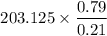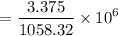Chemistry, 08.03.2021 20:10 nikitakhrabkov123
A mixture of 75 mole% methane and 25 mole% hydrogen is burned with 25% excess air. Fractional conversions of 90% of the methane and 85% of the hydrogen are achieved; of the methane that reacts, 95% reacts to form CO2 and the balance reacts to form CO. The hot combustion product gas passes through a boiler in which heat transferred from the gas converts boiler feedwater into steam.
Required:
a. Calculate the concentration of CO (ppm) in the stack gas.
b. The CO in the stack gas is a pollutant. Its concentration can be decreased by increasing the percent excess air fed to the furnace. Think of at least two costs of doing so. (Hint: The heat released by the combustion goes into heating the combustion products; the higher the combustion product temperature, the more steam is produced.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
A mixture of 75 mole% methane and 25 mole% hydrogen is burned with 25% excess air. Fractional conver...
Questions

History, 29.07.2019 20:00

Social Studies, 29.07.2019 20:00






History, 29.07.2019 20:00




Mathematics, 29.07.2019 20:00


Mathematics, 29.07.2019 20:00






Mathematics, 29.07.2019 20:00

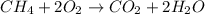
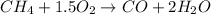
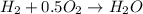
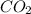 formed is = 0.95 x 67.5
formed is = 0.95 x 67.5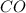 formed is = 0.05 x 67.5
formed is = 0.05 x 67.5