
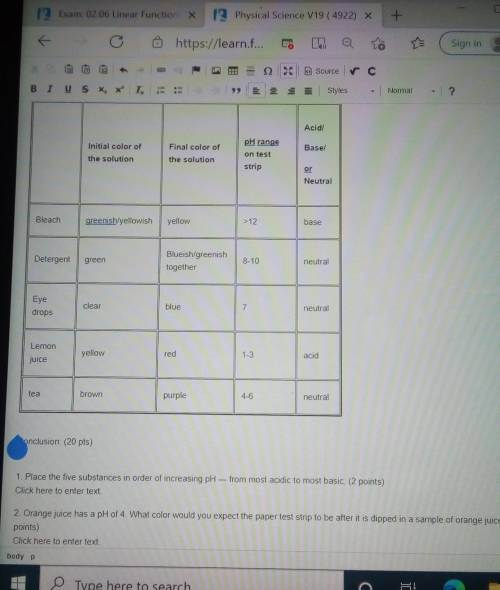
1. Place the five substances in order of increasing pH — from most acidic to most basic. (2 points) Click here to enter text. 2. Orange juice has a pH of 4. What color would you expect the paper test strip to be after it is dipped in a sample of orange juice? (2 points) Click here to enter text. 3. Determining the pH of substances such as purple grape juice and catsup using test strips can be difficult. Why? (3 points) Click here to enter text 4. Tear-free shampoos are advertised as having a pH similar to that of human tears. What pH would you expect tear-free shampoos to be? Use evidence from the lab activity to support your response. (5 points) Click here to enter text. 5. What is the approximate hydronium ion concentration and hydroxide ion concentration in a cup of tea? Which is higher? (Refer back to the chart of pH in the lesson if you need to.) (4 points) Click here to enter text. 6. What is the approximate hydronium ion concentration and hydroxide ion concentration in bleach? Which is higher? (4 points) Click here to enter text. 7 plzz help mee!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
1. Place the five substances in order of increasing pH — from most acidic to most basic. (2 points)...
Questions

Chemistry, 17.12.2020 17:00

History, 17.12.2020 17:00

Chemistry, 17.12.2020 17:00

Mathematics, 17.12.2020 17:00


History, 17.12.2020 17:00

Mathematics, 17.12.2020 17:00




World Languages, 17.12.2020 17:00

Mathematics, 17.12.2020 17:00

Mathematics, 17.12.2020 17:00


Biology, 17.12.2020 17:00

Social Studies, 17.12.2020 17:00



English, 17.12.2020 17:00

English, 17.12.2020 17:00



