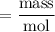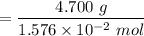Chemistry, 09.03.2021 04:30 lexibyrd120
gA scientist is trying to discover information about an unknown metal in a compound. The formula for the compound is believed to be XBr3XBr3 where XX is the unknown metal. The scientist determined that a 4.700 g4.700 g sample of this compound contains 4.834×10−2 mol Br4.834×10−2 mol Br . Calculate the atomic mass of the unknown metal, XX .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1


Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
gA scientist is trying to discover information about an unknown metal in a compound. The formula for...
Questions


Physics, 27.04.2021 22:00

Computers and Technology, 27.04.2021 22:00



Mathematics, 27.04.2021 22:00


Mathematics, 27.04.2021 22:00

Mathematics, 27.04.2021 22:00


English, 27.04.2021 22:00


Mathematics, 27.04.2021 22:00




Mathematics, 27.04.2021 22:00



Mathematics, 27.04.2021 22:00

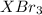
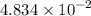 mol of Br.
mol of Br. 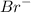 contain in 1 mol of
contain in 1 mol of 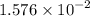 mol of
mol of 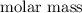 of
of