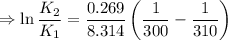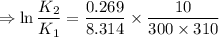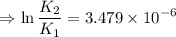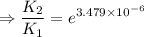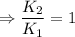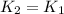Chemistry, 09.03.2021 04:40 blancademarco1994
2) A common "rule of thumb" -- for many reactions around room temperature is that the
rate will double for each ten degree increase in temperature. Does the reaction you hav
studied seem to obey this rule.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

You know the right answer?
2) A common "rule of thumb" -- for many reactions around room temperature is that the
rate will d...
Questions

Mathematics, 05.05.2020 12:20

Physics, 05.05.2020 12:20

English, 05.05.2020 12:20

Mathematics, 05.05.2020 12:20

English, 05.05.2020 12:20


Mathematics, 05.05.2020 12:20

Mathematics, 05.05.2020 12:20


Mathematics, 05.05.2020 12:20


Biology, 05.05.2020 12:20



History, 05.05.2020 12:20

Mathematics, 05.05.2020 12:20




History, 05.05.2020 12:20

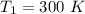
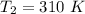
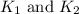 respectively.
respectively.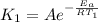 and
and 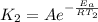
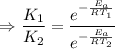
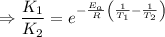
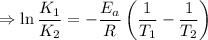
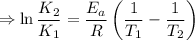
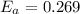 J/mol
J/mol