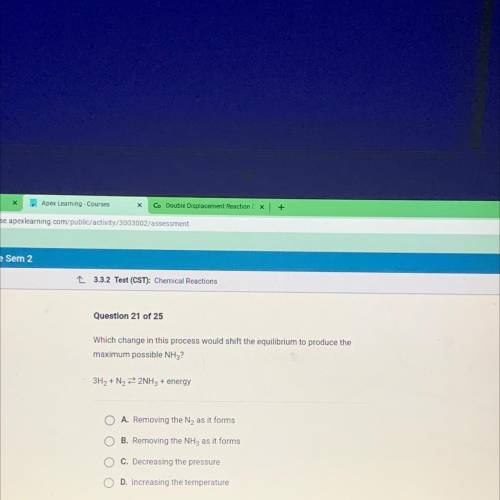
Which change in this process would shift the equilibrium to produce the
maximum possible NH3?
...

Chemistry, 09.03.2021 06:00 stressedstudent1906
Which change in this process would shift the equilibrium to produce the
maximum possible NH3?
3H2 + N2 2 2NH3 + energy
A. Removing the N2 as it forms
B. Removing the NH3 as it forms
c. Decreasing the pressure
D. Increasing the temperature

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

You know the right answer?
Questions

Mathematics, 06.04.2021 14:00

History, 06.04.2021 14:00

English, 06.04.2021 14:00


Mathematics, 06.04.2021 14:00


Mathematics, 06.04.2021 14:00

Mathematics, 06.04.2021 14:00


Mathematics, 06.04.2021 14:00

Medicine, 06.04.2021 14:00


Mathematics, 06.04.2021 14:00

Mathematics, 06.04.2021 14:00



Mathematics, 06.04.2021 14:00

Law, 06.04.2021 14:00

Physics, 06.04.2021 14:00

Mathematics, 06.04.2021 14:00



