
Chemistry, 09.03.2021 08:50 ashleyroberson735
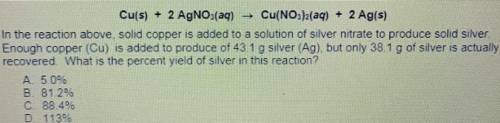
Cu(s) + 2 AgNO3(aq) Cu(NO3)2(aq) + 2 Ag(s)
14. In the reaction above solid copper is added to a solution of silver nitrate to produce solid silver.
Enough copper (Cu) is added to produce of 431 g silver (Ag) but only 38.1 g of silver is actually
recovered What is the percent yield of silver in this reaction?
A. 5.0%
B. 81.2%
C 88 46
D. 1139

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1


Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
Cu(s) + 2 AgNO3(aq) Cu(NO3)2(aq) + 2 Ag(s)
14. In the reaction above solid copper is added to a sol...
Questions

Chemistry, 02.12.2021 03:20


Mathematics, 02.12.2021 03:20

Social Studies, 02.12.2021 03:20

History, 02.12.2021 03:20

Computers and Technology, 02.12.2021 03:20

Social Studies, 02.12.2021 03:20

Chemistry, 02.12.2021 03:20

Computers and Technology, 02.12.2021 03:20

Computers and Technology, 02.12.2021 03:20

Mathematics, 02.12.2021 03:20

Mathematics, 02.12.2021 03:20




Computers and Technology, 02.12.2021 03:20


Mathematics, 02.12.2021 03:20

Advanced Placement (AP), 02.12.2021 03:20




