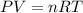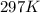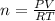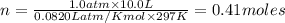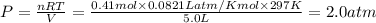Chemistry, 09.03.2021 20:10 2020davidhines
A sample of nitrogen (N2) gas in a 10.0L container has a
pressure of 1.0 atm at 297 K. Assuming ideal gas behavior,
what will the pressure be if the same amount of nitrogen
gas is put into a 5.0L container at 297 K?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

You know the right answer?
A sample of nitrogen (N2) gas in a 10.0L container has a
pressure of 1.0 atm at 297 K. Assuming ide...
Questions

Computers and Technology, 10.12.2021 19:30



Mathematics, 10.12.2021 19:30

English, 10.12.2021 19:30


Computers and Technology, 10.12.2021 19:30



English, 10.12.2021 19:30

Social Studies, 10.12.2021 19:30

Chemistry, 10.12.2021 19:30




Engineering, 10.12.2021 19:30


Computers and Technology, 10.12.2021 19:30


Biology, 10.12.2021 19:30