
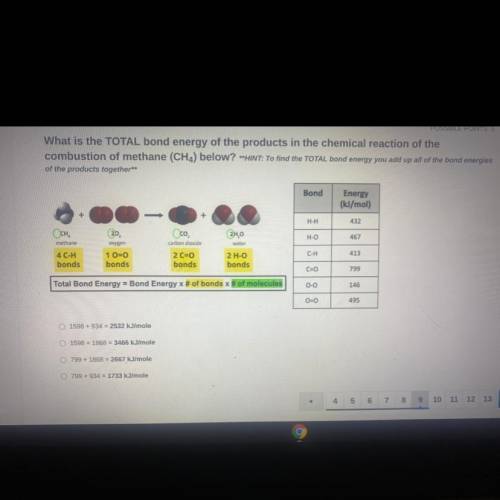
What is the TOTAL bond energy of the products in the chemical reaction of the
combustion of methane (CH4) below? **HINT: To find the TOTAL bond energy you add up all of the bond energies
of the products together**
Bond
Energy
(kJ/mol)
H-H
432
Осн.
H-O
467
methane
Oco
carbon dioxide
2 C=0
bonds
2H,0
water
2 H-O
bonds
oxygen
1 0=0
bonds
413
C-H
4 C-H
bonds
C=0
799
Total Bond Energy = Bond Energy x # of bonds of molecules
0-0
146
00
495
1598 +934 = 2532 kJ/mole
1598 +1868 = 3466 kJ/mole
799 + 1868 = 2667
kJ/mole
799 + 934 = 1733 kJ/mole

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2


Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
What is the TOTAL bond energy of the products in the chemical reaction of the
combustion of methane...
Questions

Biology, 08.11.2019 09:31

Biology, 08.11.2019 09:31

Social Studies, 08.11.2019 09:31


History, 08.11.2019 09:31

Mathematics, 08.11.2019 09:31

Social Studies, 08.11.2019 09:31

Mathematics, 08.11.2019 09:31



Computers and Technology, 08.11.2019 09:31

Physics, 08.11.2019 09:31

Mathematics, 08.11.2019 09:31

Advanced Placement (AP), 08.11.2019 09:31


Mathematics, 08.11.2019 09:31



History, 08.11.2019 09:31



