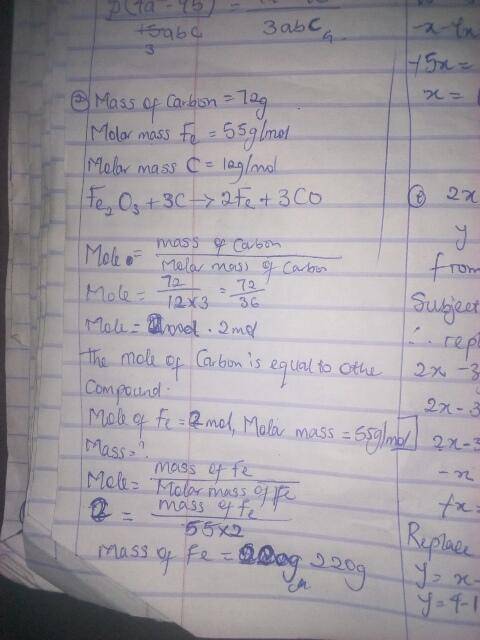
Fe2O3 + 3 C ---> 2 Fe + 3 CO
How many g Fe will form from 72 g C ?
molar mass Fe = 55...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3


Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1

Chemistry, 23.06.2019 12:30
0.070g of hydride of carbon occupies 56cm^3 at s.t.p when vaporized and contained 14.29% by mass of hydrogen.what is the formula for the hydrocarbon
Answers: 1
You know the right answer?
Questions

Mathematics, 01.11.2020 21:50






Mathematics, 01.11.2020 21:50

Biology, 01.11.2020 21:50



Mathematics, 01.11.2020 21:50

Engineering, 01.11.2020 21:50

Mathematics, 01.11.2020 21:50



Mathematics, 01.11.2020 21:50




Physics, 01.11.2020 21:50