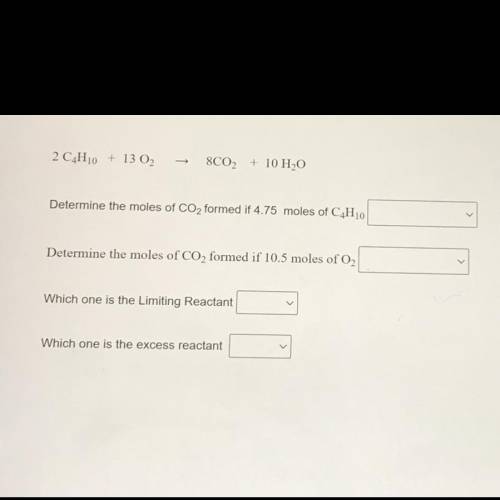
2 C4H10 + 13 02
8C02 + 10 H2O
Determine the moles of CO2 formed if 4.75 moles of C4H10
...

Chemistry, 10.03.2021 04:00 vrentadrienneoqug1a
2 C4H10 + 13 02
8C02 + 10 H2O
Determine the moles of CO2 formed if 4.75 moles of C4H10
<
Determine the moles of CO2 formed if 10.5 moles of O2
Which one is the Limiting Reactant
Which one is the excess reactant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 09:00
Which explanation is true about what happens to a ray of light when it strikes a mirror? a. a light ray is transmitted toward a mirror at a certain angle. the light ray is then reflected by the mirror at an equal angle but in the opposite direction of the transmitted ray. b. an incident ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then reflected at an angle equal to the angle of incidence but on the opposite side of the perpendicular line. c. a reflecting ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then refracted at an angle equal to the angle of the reflecting ray and on the same side of the perpendicular line. d. an incident ray strikes a mirror at an angle with a line parallel to the mirror. the light ray is then transmitted at an angle equal to the angle of incidence but on the opposite side of the parallel line. you so much! : -d take the time to try and answer correctly.
Answers: 3

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
Questions


English, 17.09.2019 08:30


Computers and Technology, 17.09.2019 08:30



Mathematics, 17.09.2019 08:30

Health, 17.09.2019 08:30

Mathematics, 17.09.2019 08:30


Mathematics, 17.09.2019 08:30



Computers and Technology, 17.09.2019 08:30





Biology, 17.09.2019 08:30

Chemistry, 17.09.2019 08:30



