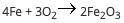the missing options are :
(1) lower boiling point and a lower freezing point(2) lower boiling point and a higher freezing point(3) higher boiling point and a lower freezing point(4) higher boiling point and a higher freezing point
answer : the correct answer is option (3) higher boiling point and a lower freezing point
explanation :
when a non volatile solute is added to a solvent like water, it results in elevation of boiling point.
let us find the increase in boiling point of water when cacl₂ is added to water to make a solution of 1 m
step 1 : find molality of the solution
let us assume we have 1 l of solution.
}{l ( solution)} )
}{1 l }= 1 mol cacl_{2} )
let us convert this to grams of cacl₂.
molar mass of cacl2 is 111 g/mol
grams of cacl₂ = 
mass of solute = 111 g
assuming density of solution same as water which is 1g/ml we have ,
mass of solution = density * volume
mass of solution = 
mass of solution = mass of solvent + mass of solute
mass of solute = 1000g - 111g
mass of solute 889 g = 0.889 kg
the molality of the solution can be calculated as
}{kg ( solvent)} )

molality = 1.12 m
step 2 : find boiling point elevation.
the elevation in boiling point is calculated as

here δtb = boiling point elevation
i = vant hoff factor which is the number of ions formed by the solute .
cacl₂ dissociates as 
it forms 3 ions . therefore,
i = 3
m = molality which is 1.12 m
kb is constant for a given solvent. for water it is 0.512


the boiling point of water increases by 1.7 °c
step 3: find freezing point depression.
when a non volatile solute is added to water, it results in decrease in freezing point.
the formula to find freezing point depression is

kf is constant for water and its value is 1.86


the freezing point of water decreases by 6.2 °c
therefore we can say that, the solution will have a higher boiling point and a lower freezing point