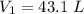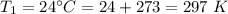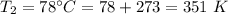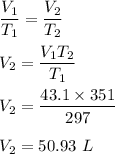Chemistry, 10.03.2021 06:50 leylaanddade
Enter your answer in the provided box. A 43.1−L volume of methane gas is heated from 24°C to 78°C at constant pressure. What is the final volume of the gas?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Enter your answer in the provided box.
A 43.1−L volume of methane gas is heated from 24°C to 78°C a...
Questions

Social Studies, 03.02.2021 17:00

Arts, 03.02.2021 17:00

Mathematics, 03.02.2021 17:00



Social Studies, 03.02.2021 17:00


History, 03.02.2021 17:00



Mathematics, 03.02.2021 17:00

Computers and Technology, 03.02.2021 17:00

Biology, 03.02.2021 17:00

Mathematics, 03.02.2021 17:00

Computers and Technology, 03.02.2021 17:00

Mathematics, 03.02.2021 17:00