Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
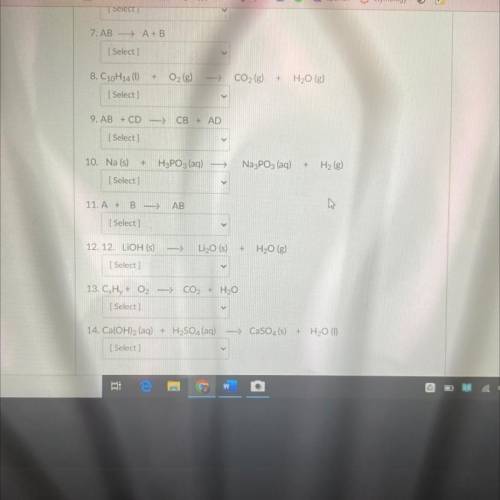
For each of the following reactions choose from the options below: (Reactions do not need to balance...
Questions

Biology, 02.12.2020 01:00



Mathematics, 02.12.2020 01:00


Biology, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00

English, 02.12.2020 01:00

Biology, 02.12.2020 01:00

History, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00





Mathematics, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00

Biology, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00

Biology, 02.12.2020 01:00