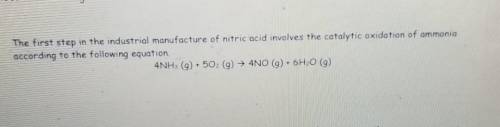How Many moles of oxygen(02) are needed to produce 4.6 g of nitrogen monoxide (NO)?
3.36 mol
...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1


Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
Questions


Chemistry, 16.02.2021 09:00


Social Studies, 16.02.2021 09:00

Mathematics, 16.02.2021 09:00

Mathematics, 16.02.2021 09:00

Biology, 16.02.2021 09:00



Mathematics, 16.02.2021 09:00

Mathematics, 16.02.2021 09:00

Mathematics, 16.02.2021 09:00

History, 16.02.2021 09:00

Mathematics, 16.02.2021 09:00


Mathematics, 16.02.2021 09:00

Business, 16.02.2021 09:00

Mathematics, 16.02.2021 09:00

French, 16.02.2021 09:00