Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2

Chemistry, 23.06.2019 13:30
Which of these statements describes the size of an atom? a. an atom is larger than a sheet of aluminum foil. b. an atom is small but can be seen with just our eyes. c. an atom is the size of a plastic building block. d. an atom is tiny and cannot be seen without magnification.
Answers: 2
You know the right answer?
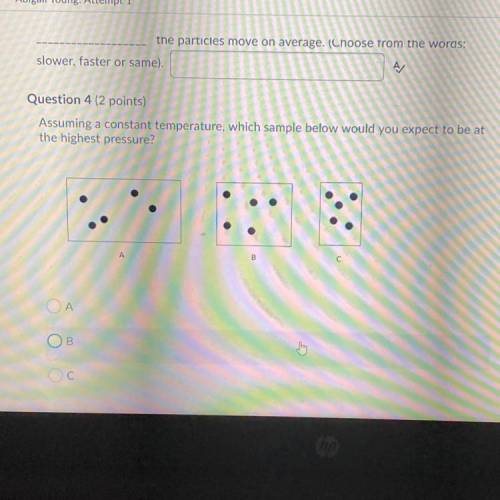
Assuming a constant temperature, which sample below would you expect to be at
the highest pressure?...
Questions



English, 17.07.2019 13:00



Biology, 17.07.2019 13:00

Computers and Technology, 17.07.2019 13:00

Advanced Placement (AP), 17.07.2019 13:00


Mathematics, 17.07.2019 13:00

History, 17.07.2019 13:00

History, 17.07.2019 13:00



Geography, 17.07.2019 13:00

Chemistry, 17.07.2019 13:00

Spanish, 17.07.2019 13:00



History, 17.07.2019 13:00