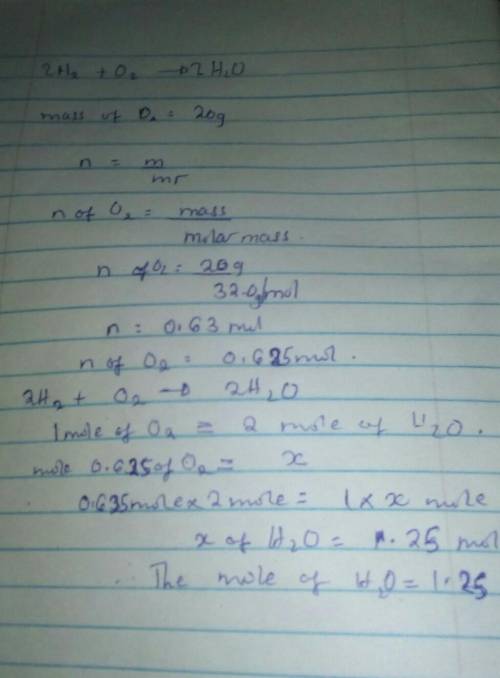Chemistry, 10.03.2021 23:00 trinidymwilga
Question 8 (1 point) 2H₂ + O₂ O2 --> 2 H20 Molar mass of H2 = 2.0 O2 =32.0 H2O=18.0 If you start with 20 grams of Oz, how many moles of H2O can be made? 1.25 mol 320 mol 1280 mol 0.31 mol Question 9 (1 point)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1


Chemistry, 23.06.2019 12:00
372 ml is the volume of aluminum, density is 2.70 g/ml what is the mass in grams
Answers: 1

Chemistry, 23.06.2019 13:30
Asap at one point in history people could measure temperature by looking at the volume of a sample of gas. suppose a sample in a gas thermometer has a volume of 135ml at 11.0°c. indicate what temperature would correspond to each of the following volumes: 113 ml, and 155 ml.
Answers: 1
You know the right answer?
Question 8 (1 point) 2H₂ + O₂ O2 --> 2 H20 Molar mass of H2 = 2.0 O2 =32.0 H2O=18.0 If you start...
Questions


Mathematics, 17.03.2020 17:37

Mathematics, 17.03.2020 17:38


Chemistry, 17.03.2020 17:38

Mathematics, 17.03.2020 17:38

Social Studies, 17.03.2020 17:38


Biology, 17.03.2020 17:38

English, 17.03.2020 17:38


Mathematics, 17.03.2020 17:38

Mathematics, 17.03.2020 17:38

English, 17.03.2020 17:38


Physics, 17.03.2020 17:39

Mathematics, 17.03.2020 17:39

Mathematics, 17.03.2020 17:39

Mathematics, 17.03.2020 17:39

Mathematics, 17.03.2020 17:39