
Chemistry, 11.03.2021 05:30 feliciagraham14
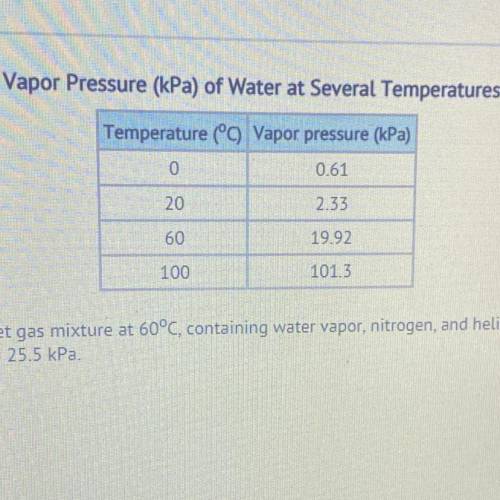
What is the total pressure of a wet gas mixture at 60°C, containing water vapor, nitrogen, and helium. The partial pressures are Pnitrogen = 53.0 kPa and Phelium = 25.5 kPa.
A
58.58 kPa
B)
78.50 kPa
C)
98.42 kPa
D
101.32 KP

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1



Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
What is the total pressure of a wet gas mixture at 60°C, containing water vapor, nitrogen, and heliu...
Questions


Social Studies, 22.06.2019 10:00



Spanish, 22.06.2019 10:00

Biology, 22.06.2019 10:00

Chemistry, 22.06.2019 10:00


Mathematics, 22.06.2019 10:00

Mathematics, 22.06.2019 10:00




History, 22.06.2019 10:00



Mathematics, 22.06.2019 10:00

History, 22.06.2019 10:00




