Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
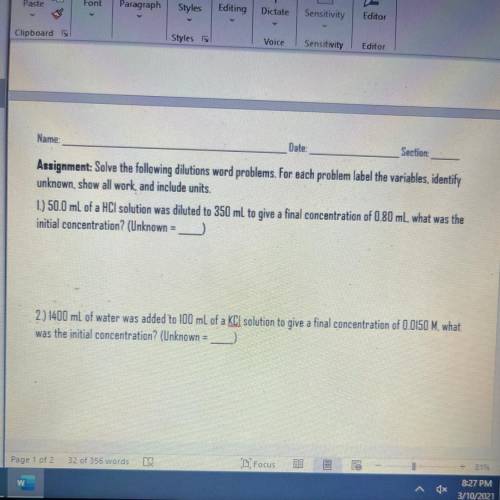
1.) 50.0 mL of a HCl solution was diluted to 350 mL to give a final concentration of 0.80 ml, what w...
Questions

English, 25.06.2019 01:10


Chemistry, 25.06.2019 01:10





Mathematics, 25.06.2019 01:10



Mathematics, 25.06.2019 01:10

Mathematics, 25.06.2019 01:10

English, 25.06.2019 01:10



English, 25.06.2019 01:10

Mathematics, 25.06.2019 01:10


Mathematics, 25.06.2019 01:10

Physics, 25.06.2019 01:10