
Chemistry, 11.03.2021 05:50 ilovemusicandreading
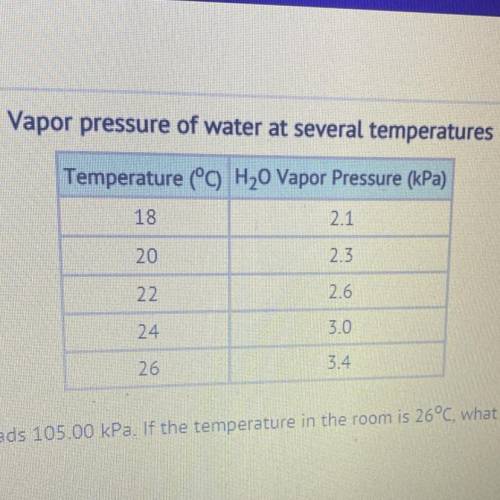
The barometer at an indoor pool reads 105.00 kPa. If the temperature in the room is 26°C, what is the partial pressure of the dry
air??
A
30.88 kPa
B)
101.60 kPa
108.40 kPa
D)
357.00 kPa

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1

Chemistry, 23.06.2019 10:30
4al + 3o2 → 2al2o3 what does the "3" in front of o2 stand for? a) it indicates that there are 5 oxygen atoms after you add the coefficient and the subscript. b) it indicates that that there are are total of 6 oxygen atoms all bonded together as a single molecule. c) it indicates that there are 3 oxygen molecules chemically bonded to each other in the reaction. d) it indicates that there are 3 separate oxygen molecules in the reaction.
Answers: 2
You know the right answer?
The barometer at an indoor pool reads 105.00 kPa. If the temperature in the room is 26°C, what is th...
Questions

Mathematics, 29.04.2021 14:00

Physics, 29.04.2021 14:00

Chemistry, 29.04.2021 14:10


Biology, 29.04.2021 14:10

Mathematics, 29.04.2021 14:10

Mathematics, 29.04.2021 14:10

Mathematics, 29.04.2021 14:10

English, 29.04.2021 14:10

English, 29.04.2021 14:10

History, 29.04.2021 14:10

History, 29.04.2021 14:10

Geography, 29.04.2021 14:10


Computers and Technology, 29.04.2021 14:10



English, 29.04.2021 14:10


English, 29.04.2021 14:10




