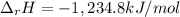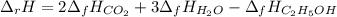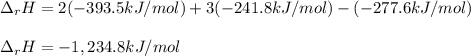Alcohol is used as a source of fuel for some automobiles. The standard molar enthalpy of formation of ethanol C2H5OH(l) is -277.6 kJ/mol. For the balanced reaction equation of ethanol use:
C2H5OH(l) + 3O2 --> 2CO2 + 3H2O.
Ethanol is a liquid, but all the other chemicals in this reaction are gases. What is the enthalpy change of this reaction in kJ/mol?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1
You know the right answer?
Alcohol is used as a source of fuel for some automobiles. The standard molar enthalpy of formation o...
Questions




Mathematics, 18.03.2020 16:34





Biology, 18.03.2020 16:35

Computers and Technology, 18.03.2020 16:35



Computers and Technology, 18.03.2020 16:36

Mathematics, 18.03.2020 16:36


Mathematics, 18.03.2020 16:36

Biology, 18.03.2020 16:36

Mathematics, 18.03.2020 16:36