
Chemistry, 11.03.2021 14:10 kaelynnmarie1135
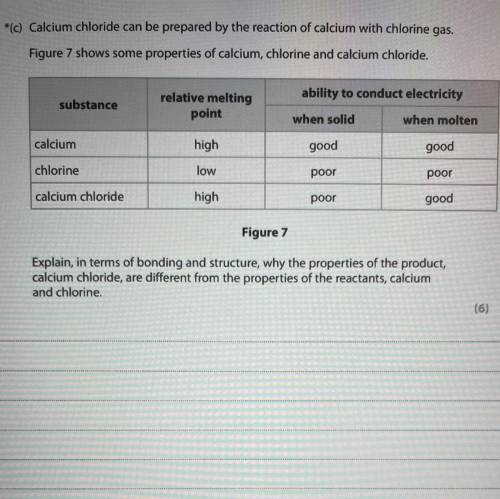
Calcium chloride can be prepared by the reaction of calcium with chlorine gas.
Figure 7 shows some properties of calcium, chlorine and calcium chloride.
ability to conduct electricity
substance
relative melting
point
when solid
when molten
calcium
high
good
good
chlorine
low
poor
poor
calcium chloride
high
poor
good
Figure 7
Explain, in terms of bonding and structure, why the properties of the product,
calcium chloride, are different from the properties of the reactants, calcium
and chlorine.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
Calcium chloride can be prepared by the reaction of calcium with chlorine gas.
Figure 7 shows some...
Questions

Geography, 23.02.2021 01:50



Health, 23.02.2021 01:50






Social Studies, 23.02.2021 01:50




Mathematics, 23.02.2021 01:50

Mathematics, 23.02.2021 01:50


Spanish, 23.02.2021 01:50





