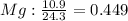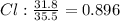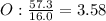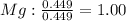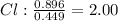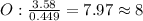Chemistry, 07.01.2020 23:31 wsdafvbhjkl
An ionic compound is found to contain 10.9% magnesium, 31.8% chlorine and 57.3% oxygen. what is the empirical formula for this compound?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1
You know the right answer?
An ionic compound is found to contain 10.9% magnesium, 31.8% chlorine and 57.3% oxygen. what is the...
Questions

Mathematics, 14.01.2021 22:10


Chemistry, 14.01.2021 22:10







History, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10


Mathematics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

Social Studies, 14.01.2021 22:10

Physics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10

Mathematics, 14.01.2021 22:10


Mathematics, 14.01.2021 22:10