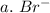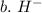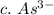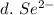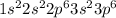a. fe

Chemistry, 02.02.2020 21:47 jazzycintron14
1.* write electron configurations for the 2 + 2 plus cations of these elements.
a. fe
b. co
c. ni
2.write electron configurations for the 3 + 3 plus cations of these elements.
a. chromium
b. manganese
c. iron
3. write the symbol for the ion formed when each element gains electrons and attains a noble-gas electron configuration.
a. br
b. h
c. as
d. se
4.* write electron configurations for the following atoms and ions, and comment on the result.
ar
cl − cap cl to the minus
s 2 − cap s super 2 minus end super
p 3 −

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3

Chemistry, 23.06.2019 21:10
Which of the following characteristics are true about a typical peptide (amide) bond? the bond is planar. there is free rotation about the carbonyl carbon and nitrogen bond. there is substantial double-bond character to this bond. there is a net negative charge on nitrogen and net positive charge on oxygen.
Answers: 3
You know the right answer?
1.* write electron configurations for the 2 + 2 plus cations of these elements.
a. fe
a. fe
Questions

Geography, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00

Geography, 11.07.2019 02:00


Geography, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00



Health, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00



Chemistry, 11.07.2019 02:10

Mathematics, 11.07.2019 02:10

History, 11.07.2019 02:10

Biology, 11.07.2019 02:10

Mathematics, 11.07.2019 02:10

Chemistry, 11.07.2019 02:10

![a.\ Fe^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s 3d^{5} \ or \ [Ar]4s 3d^{5}](/tpl/images/0494/0749/a44c8.png)
![b.\ Co^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{5} \ or \ [Ar]4s^{2} 3d^{5}](/tpl/images/0494/0749/d425b.png)
![c.\ Ni^{2+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{6} \ or \ [Ar]4s^{2} 3d^{6}](/tpl/images/0494/0749/26975.png)
![a.\ Cr^{3+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d \ or \ [Ar]4s^{2} 3d](/tpl/images/0494/0749/4de3e.png)
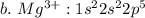
![c.\ Fe^{3+}: 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{6} 4s^{2} 3d^{3} \ or \ [Ar]4s^{2} 3d^{3}](/tpl/images/0494/0749/51e46.png)